Tooth samples
A total of 54 teeth extracted for orthodontic purposes were used for the experiments. All teeth used in the experiments were undamaged, unbleached molars, with no signs of caries or other diseases. The samples were obtained from nonsmoking women and men aged 25â45 years. Sampling and further experimentsâ procedure was approved by Bioethics Committee at the District Medical Chamber in Krakow, Poland (approval number: 154/KBL/OIL/2016). All methods were performed in accordance with the guidelines and regulations provided by the Committee. As agreed, teeth were granted by the Department of Dental Surgery at the University Dental Clinic in Krakow (Poland), after patientsâ informed consent. After extraction, the teeth were stored in formalin (10% p. Chempur, Piekary ÅlÄ skie Poland). They were then cleaned of soft tissues with 2% papain (Merck, Darmstadt, Germany), deionized water, and fluoride toothpaste. After the samples were thoroughly rinsed, they were dried in a laboratory oven (Heratherm; Thermo Fisher Scientific, MA, USA) for 16 h at 60 °C and stored in this form in a refrigerator (4 °C) until the experiments were conducted. In total, 4 experiments were performed. Details of the experimental setups are presented in “Experiments“.
Orthodontic appliances
Three sets of orthodontic braces were used to conduct the experiments. Each set consisted of the following: 20 brackets, 4 bands (size: 37), and 2 NiâTi or NiâCr archwires. Please refer to Supplementary Table S3 for manufacturing details. The experiments used typical, commercially available, mid-priced sets of braces. The orthodontic archwires were replaced in the reactors every 30 cycles simulating daily changes in oral pH.
Solutions and reagents
As part of the experiments, the solutions in the experimental reactors were changed daily. The solutions were prepared according to the standard recipe used in pH cycling experiments56. Remineralizing solution composition: 1.5 mmol CaCl2âÃâ2 H2O p.a. (Chempur, Piekary ÅlÄ skie Poland), 0.9 mmol KH2PO4 p.a. (Chempur, Piekary ÅlÄ skie Poland), 130 mmol KCl p.a. (Chempur, Piekary ÅlÄ skie Poland), 20 mmol HEPES bufor p.a. (4-(2-hydroxyethyl)-1-piperazine ethane sulfonic acid) (CARL ROTH, Karlsruhe, Germany), and KOH p.a. to adjust pHâ7.0 (Chempur, Piekary ÅlÄ skie Poland). Demineralizing solution composition: 1.5 mmol CaCl2âÃâ2 H2O p.a. (Chempur, Piekary ÅlÄ skie Poland), 0.9 mmol KH2PO4 p.a. (Chempur, Piekary ÅlÄ skie Poland), acetic acid p.a. (50 mmol), and KOH p.a. to adjust pHâ4.3 (Chempur, Piekary ÅlÄ skie Poland).
Fluoride (0.0047 mmol NaF 99+% (Acros Organics, Thermo Scientific Chemical, Delphi, India)), was added to the remineralization solution as it plays a crucial role in both enamel remineralization and metal corrosion24,25,33. The solutions were prepared using deionized water with a conductivity of 5 µS/cm produced by a R5Uv device (Hydrolab, Straszyn, Poland). In addition, a commercially available oral hygiene liquid (Elmex; Colgate-Palmolive, Åwidnica, Poland), recommended for oral hygiene during orthodontic treatment, was used in the daily pH cycles. The pH of the fluid, measured using a CPI-505 device with an EPS-1 electrode (Emeltron, Zabrze, Poland), was 4.50â±â0.05.
The daily sequence of the solutions in the reactors was as follows: step 1âdemineralization (30â45Â min); step 2âhygiene: mouthwash (2Â min), tap water (10Â s), deionized water (10Â s); step 3âremineralization (3â6Â h); step 4âdemineralization (30â45Â min); step 5âhygiene: mouthwash (2Â min), tap water (10Â s), deionized water (10Â s); step 6âremineralization (16â19.5Â h). Demineralization and remineralization solutionsâ volume was 100Â ml, while mouthwash solution volume was 50Â ml. The proportions of remineralization and demineralization time were determined by taking into account the abiotic nature of the experiment (lack of enzymes and organic constituents protecting the enamel against dissolution)42. The pH cycle has been designed to prevent excessive demineralization of the enamel on the one hand and to maintain the acid shock occurring in the oral cavity, which causes leakage of metals from the appliance on the other hand25,29. Daily changes in the concentration of Ca in the experimental solutions resulting from element release (demineralization) or uptake (remineralization) were balanced (Supplementary Fig. S2). On the other hand, the amounts of metals released from the parts of orthodontic appliance (Supplementary Fig. S3) were within the range of previously published data32.
Experiments
Total concentrations of metals in enamel
This part of the research consisted of three complimentary experiments: Experiment #1 (the effect of the presence of appliances in the solutions on the enamel content of: Fe, Cr, Ni, and Cu); Experiment #2 (the effect of the applied solutionsâ pH cycles on the enamel content of: Fe, Cr, Ni, and Cu); Experiment #3 (the influence of dividing teeth into sample halves on the enamel content of: Fe, Cr, Ni, and Cu). In each experiment, there was a main (experimental) and control group. The details of the experimental setup are presented in Table 1 and in Supplementary Fig. S1. The Experiments #2 and #3 were conducted to ensure that the potential differences in enamel metal content between the main group and the control group in the Experiment #1 were solely a result of the presence of the appliance and not of the experimental procedures or of the inhomogeneous nature of the enamel.
To prepare the experimental samples, 53 out of the 54 extracted and pre-prepared teeth (see “Tooth samples“) were cut into corresponding halves labelled as âAâs and âBâs. This resulted in total 106 enamel samples, which were arranged for the Experiment #1, Experiment #2, Experiment #3 and their controls. The âAâ tooth halves were used in the main groups, whereas their corresponding âBâ halves were arranged as the control samples. Cutting along the lingualâbuccal cross-section was performed with a water-cooled dental separator (DFS-DIAMO, Riedenburg, Germany). The exposed tooth cross-sections were secured with stickers (Color Coding Dots 3010; Avery Dennison Zweckform, München-Grünwald, Germany). This method effectively protects the enamel surface in experimental studies using a pH cycle, which has been confirmed previously55. The stickers were replaced every 15 solution cycles (for details see below).
The Experiment #1 involved 70 enamel samples. 35 tooth halves (A-s) of the main, experimental group (labelled as: 1A) and 35 corresponding tooth halves (B-s) of the control group (labelled as: 1B) were placed in the 100Â ml high-density polypropylene (HDPP) reactors. In total, there were six reactors in this experiment, i.e., three reactors for each group with: 12, 12 and 11 samples in each of them, respectively. Reactors of the experimental group additionally contained orthodontic appliance as indicated in Table 1. The solutions in each reactor were changed daily according to the sequence described in “Solutions and reagents“. The reactors were disinfected once a day with disinfectant solution (Pursept A Xpress; Schulke, Norderstedt, Germany) and thoroughly washed with deionized water. The samples were rinsed in deionized and tap water on polypropylene sieves: the contents of a given reactor were placed in the assigned sieve and rinsed under a running stream of deionized or tap water while shaking vigorously. Once a week, the samples were disinfected with disinfectant solution (Pursept A Xpress; Schulke, Germany). This applied procedure allowed long-term experiments to be conducted in a continuous mode without biofilm contamination of reactors, samples, and appliances.
The experiments were conducted for 18 months, during which a total of 360 rinsing cycles were performed. For technical breaks, the samples were placed in a laboratory refrigerator at temperature 4 °C (Whirlpool, WrocÅaw, Poland) in reactors filled with remineralizing solution to cover the tooth samples17,29. This lowered the kinetics of the de and remineralization reactions (Supplementary Fig. S2). During the experiments, the reactors were placed in an incubator at 36.6 °C and shaken at 80 RPM (GLF 3033; Gesellschaft für Labortechnik, Burgwedel, Germany). The reactors were covered with a polypropylene lid, enabling air exchange and protecting against evaporation. After the experiments, the enamel samples were rinsed thoroughly with tap water (2 min) and deionized water (2 min), disinfected with Pursept A Xpress (Schulke, Norderstedt, Germany) solution, and placed in a refrigerator (4 °C) prior to metal concentration analysis using ICPâMS (details described in “Analytical methods“).
The Experiment #2 involved 16 samples. The main, experimental group (labelled as 2Aâ) involved 8 tooth halves (Aâ-s), whereas the control group (labelled as 2Bâ) arranged remaining corresponding 8 tooth halves (Bâ-s). Samples were placed in the 100 ml HDPP reactors covered with a polypropylene lid. In total, there were two reactors in this experiment, i.e., one for each group with 8 enamel samples in it. Similarly to the group 2B of the Experiment #1, the enamel from the main group (2Aâ) of the Experiment #2 undergone 360 pH cycles (during 18 months), at 36.6 °C and 80 RPM (GLF 3033; Gesellschaft für Labortechnik, Burgwedel, Germany). The procedures for the solution sequence as well as the maintaining of the tooth samples and the reactors were identical to those in Experiment #1. The samples from the control group (2Bâ) of the Experiment #2 were placed in the HDPP reactor in a laboratory refrigerator at 4 °C (Whirlpool, WrocÅaw, Poland) for the whole experimental time (18 months). After the experiments, the enamel samples were rinsed thoroughly with tap water (2 min) and deionized water (2 min), disinfected with Pursept A Xpress (Schulke, Norderstedt, Germany) solution, and placed in a refrigerator (4 °C) prior to metal concentration analysis using ICPâMS (details described in “Analytical methods“).
The Experiment #3, involved 20 samples. The main, experimental group (labelled as 3Aââ) consisted of ten tooth halves (Aââ-s) and the control group (labelled as 3Bââ) arranged corresponding ten tooth halves (Bââ-s). The enamel samples from both 3Aââ and 3Bââ groups were rinsed thoroughly with tap water (2 min) and deionized water (2 min), disinfected with Pursept A Xpress (Schulke, Norderstedt, Germany) solution, and placed in a refrigerator (4 °C) prior to metal concentration analysis using ICPâMS (details described in “Analytical methods“). There was no pH cycling involved in this experiment.
Spatial distribution of metals in enamel before and after orthodontic treatment simulation (Experiment #4)
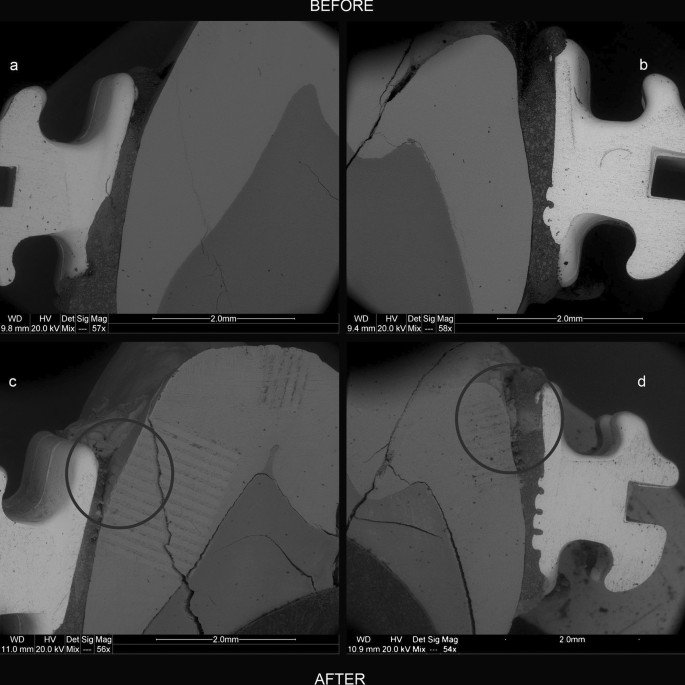
For this experiment, one tooth was prepared differently than the other 53 samples. After washing and cleaning the soft tissues (description in “Tooth samples“), two brackets from the set used in the reactor 3e of Experiment #1 (Table 1) were adhered to the tooth surface on its buccal and lingual sides using the Transbond TM XT kit (3M Unitek, CA, USA). The procedure used was in accordance with the adhesive system manufacturerâs guidelines. Phosphoric acid (36%) (ARKONA, Niemce, Poland) was used as the etching agent.
Then, using sandpaper made of SiC and 1 micron Poly-Top-DUO Diamond slurry (Microdiamant, Lengwil, Switzerland), the lingualâbuccal cross-section of the tooth was exposed and analysed at selected sites using a scanning electron microscope with an energy dispersive spectrometer (SEMâEDS) and a laser ablation inductively coupled plasma mass spectrometer (LAâICPâMS). For analytical details please see “Analytical methods“. After the analyses, the exposed cross-section was secured with stickers (Color Coding Dots 3010; Avery Dennison Zweckform, München-Grünwald, Germany). This method effectively protects the enamel surface in experimental studies using a pH cycle and has been confirmed previously55. The stickers were replaced every 15 solution cycles.
The tooth was placed in Reactor 3e of the Experiment #1 (Table 1), along with the samples of the 1A group. In this reactor, the tooth underwent 360 cycles of pH changes, similar to the samples of Experiment #1 placed in the same reactor (see “Total concentrations of metals in enamel“). After termination of the pH cycles, the tooth was rinsed thoroughly with tap water (2 min) and deionized water (2 min). The exposed tooth cross-section was polished with 1 micron Poly-Top-DUO Diamond slurry (Microdiamant, Lengwil, Switzerland) to a depth of approximately 50 µm and then cleaned again with tap and deionized water and a cotton swab soaked in concentrated nitric acid (65% ultrapure; Merck, Darmstadt, Germany).
The sample prepared in this way was reanalysed using LA-ICPâMS at the same sites of the cross-section as before the experiments and then imaged using SEMâEDS. For analytical details please see “Analytical methods“. In total, 12,000 quantitative measurement points were made (Supplementary Table S5). The narrow fissures in the enamel and its contact points with the adhesive system and metal parts of the appliance were analysed particularly thoroughly.
Analytical methods
Imaging of the dental samples and analysis of the alloy composition of the parts of the orthodontic appliances were performed using a variable pressure field emission scanning electron microscope coupled with an FEI Quanta 200 FEG energy dispersive spectrometer (Thermo Fisher Scientific, OR, USA) at 20Â kV (SEMâEDS).
The analysis of the total metal concentrations in the enamel was performed using ICPâMS with an iCAP RQ (C2) instrument (Thermo Fischer Scientific, OR, USA) after complete dissolution of 0.05Â g of enamel in HNO3 acid (65% ultrapure; Merck, Darmstadt, Germany ), followed by dilution with deionized water at a ratio of 1:14. Prior to dissolution, the enamel from each tooth sample was separated from the dentin using a dental drill (#LOT: 582703; Emil Lange Zahnbohrefabrik, Engelskirchen, Germany) and then washed with deionized water and a cotton swab soaked in concentrated HNO3 (65% ultrapure, Merck, Darmstadt, Germany) to remove residual fine drill debris and nonenamel parts. The enamel from each sample was then ground with a jade mortar and pestle, averaged using the cone method, and weighed. An NIST SRM 1400 standard (SigmaâAldrich, Merck, Steinheim am Albuch, Germany) was used as reference material in the analysis. Ti was not analysed in this experiment for technical reasonsâthere were isobaric and polyatomic interferences during ICPâMS determination of titanium caused by the presence of high concentrations of Ca and P in the analysed solutions. Supplementary Table S4 shows the detection limits for the analysed elements.
The analysis of the spatial distribution of metals in the enamel was performed using LAâICPâMS. For this purpose, an ICPâMS NexION 300 spectrometer (Perkin Elmer, MA, USA) coupled with an LSX-213 laser ablation system (CETAC, NE, USA) was used. The operating parameters of the ICPâMS measurement system and laser ablation conditions are listed in Supplementary Table S6. NIST SRM 1400 and NIST SRM 610 (SigmaâAldrich, Merck, Steinheim am Albuch, Germany) were used as reference materials. The selection of the reference material for the quantitative analysis of enamel using the LA-ICPâMS method was described in our previous work56. Additionally, the method of making maps of element distribution has been described in previous publications57,58,59. Supplementary Table S7 shows the detection limits for the analysed elements.
Statistics
TIBICO Statistica version 13.3. and 14.0.0.15 (TIBICO, Palo Alto, CA, USA) was used for all statistical analyses in this study. The results of the metal concentrations in the powdered enamel samples were tested for a normal distribution using the ShapiroâWilk test, which denied a normal distribution. Therefore, the nonparametric multiple comparisons (2-tailed) of independent variables based on the KruskalâWallis test and median tests were used for further interpretation.
The results of the Experiment #1 were tested for correlations between the total concentrations of particular metals in enamel samples, using the Spearmanâs rank order correlation tool, with a significance level of pâ<â0.050. The correlations between the concentrations of Fe, Ni, Cr and Cu in the samples of the group 1A (Experiment #1) have been tested as well as the trends in metals content between groups 1A and 1B of the Experiment#1. Results were presented in Supplementary Tables S2a and S2b.
Statement of ethics
This study protocol was reviewed and approved by the Bioethics Committee at the District Medical Chamber in Krakow, approval number [154/KBL/OIL/2016]. The Department of Dental Surgery at the University Dental Clinic in Krakow supervised the study participants in terms of the collection of their teeth for the experiments. Teeth were extracted for orthodontic purposes after written informed consent was obtained.