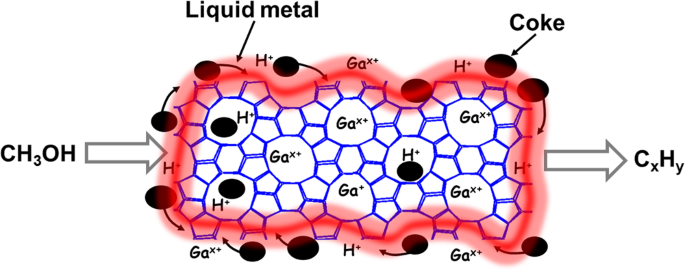
Enhancing MTH catalytic stability by liquid metal
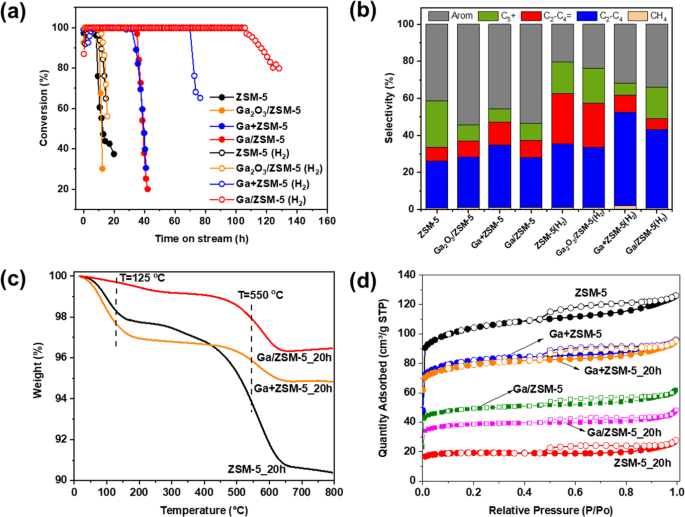
The catalytic performances of the parent ZSM-5 and Ga with ZSM-5 zeolites in the MTH reaction are illustrated in Fig. 2. The reference ZSM-5 and Ga2O3/ZSM-5 catalysts have shown a similar lifetime (defined as a period during which the methanol conversion remains stable at nearly 100%) of 8â9âh (Fig. 2a). In contrast, both the Ga+ZSM-5 prepared by physically mixing ZSM-5 with metallic gallium and Ga/ZSM-5 prepared by heat treatment of ZSM-5 with gallium have demonstrated a substantially improved catalytic stability with a lifetime of ~32âh.
Catalytic conversion (a) and selectivity to the products after 8âh of reaction (b) of ZSM-5, Ga2O3/ZSM-5, Ga+ZSM-5 and Ga/ZSM-5 in the methanol-to-hydrocarbon reaction. Reaction conditions: 400â°C, catalyst containing 50âmg of ZSM-5, 1.4âg methanolâgâ1ZSM-5âhâ1. TGA analysis (c) and low-temperature N2 adsorption (d) of initial and after 20âh on stream ZSM-5, Ga+ZSM-5, and Ga/ZSM-5.
At the initial period after about 8âh of testing, the main products formed over ZSM-5 were light paraffins (27%), C5+ hydrocarbons (20%) and aromatics (42%) with a small contribution from light olefins (7%). Ga2O3/ZSM-5 shows a higher selectivity to aromatics and a lower selectivity towards C5+ hydrocarbons in agreement with an earlier publication3 demonstrating a promoting effect of Ga oxide towards aromatization reactions. In contrast, the presence of liquid Ga in the reactor for both Ga+ZSM-5 and Ga/ZSM-5 increases the catalyst selectivity to light olefins and aromatics with a decrease in the formation of C5+ hydrocarbons as compared with pure ZSM-5. The catalytic performance changes over time with an increase in the production of olefins and a decrease in the production of light paraffins and aromatics due to a decrease in the hydrogen transfer activity of the catalysts (Fig. S1, SI). It has to be noted that Ga in the mixture with inert silica does not provide catalytic activity in methanol conversion.
The catalytic performance of ZSM-5-based catalysts in MTH with hydrogen co-feeding is also presented in Fig. 2. ZSM-5 and Ga2O3/ZSM-5 have shown insignificant effects of hydrogen on the catalyst lifetime, which is due to the use of atmospheric pressure. Indeed, high hydrogen pressure is required for the hydrogenation of carbon species inducing the deactivation of the catalyst12. In contrast, Ga+ZSM-5 and Ga/ZSM-5 have demonstrated significantly improved catalytic stability with little deactivation up to ~70 and 110âh of time-on-stream, respectively. In addition, compared to ZSM-5, the selectivity to aromatics is higher over Ga+ZSM-5 and Ga/ZSM-5, while a larger amount of paraffin is formed due to the hydrogenation reactions.
The effect of other liquid metals and alloys on the catalytic performance of ZSM-5 has been tested in the presence of indium (In), bismuth-indium (BiIn) and bismuth-tin (BiSn) alloys with melting points at 156, 62, and 138â°C, respectively (Fig. S2, SI). There is almost no effect of In on the catalyst lifetimes. BiIn and BiSn have increased the MTH stability from 8âh to 19 and 20âh, respectively. The presence of hydrogen did not affect significantly the catalytic stability of BiSn+ZSM-5 in comparison with Ga+ZSM-5 (Fig. S2, SI).
The zeolite changes color from white to gray after treatment with liquid metal such as Ga with small droplets of Ga not absorbed by zeolite (Fig. S3, SI). Ga is changing catalyst color by interaction with zeolite, however, it is still in the form of metal covered by a thin oxide layer. The presence of Ga in the metal state can be confirmed by TG analysis, where it shows an endothermic peak during melting at 33â°C (Fig. S4, SI). In the case of zeolite mixed with Ga and deposited in the reactor, metallic Ga on the wall of the reactor can be observed after the reaction (Fig. S5, SI). The chemical analysis of Ga/ZSM-5 and Ga+ZSM-5 separated from Ga droplets by sieving shows that they contain 24 and 14.9 wt. % Ga, respectively. The higher metal loading and more uniform distribution of Ga in Ga/ZSM-5 could explain the higher stability of the material in the reaction in comparison with Ga+ZSM-5. It should be noted that Ga+ZSM-5 exhibited the highest Ga content after reaction and sieving off bulk gallium droplets, surpassing other metal- and alloy-promoted zeolites (with In at 0.1 wt.%, BiSn at 0.9 wt.%, and BiIn at 5.1 wt.%). This is in line with the assumption about the stronger effect of Ga on the catalytic performance in the MTH reaction (Fig. S6, SI).
The regeneration of deactivated zeolite by calcination in air is traditionally used in the MTH process. In the case of Ga/ZSM-5 catalyst, the calcination in the air could oxidize Ga to Ga2O3 with a loss of enhanced catalytic stability. It requires additional reduction treatment to reduce oxidized gallium back to the metallic state. Our results show that the deactivated Ga/ZSM-5 catalysts can be regenerated in 3 reaction cycles without losing the catalytic performance (Fig. S7, SI).
Role of liquid Ga in improving the zeolite stability
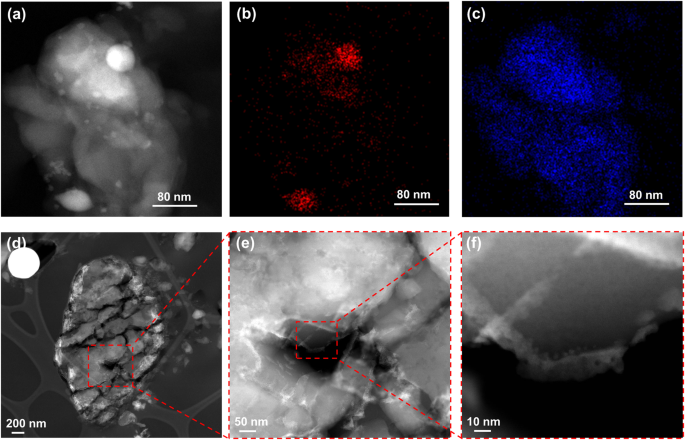
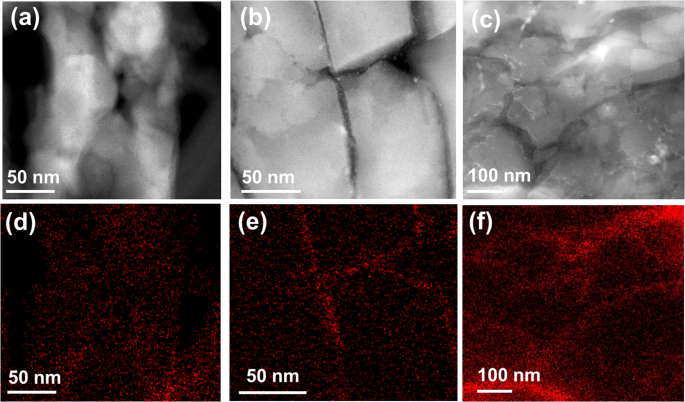
To understand the promotion effect of liquid Ga for ZSM-5 in the MTH, ZSM-5, Ga+ZSM-5, and Ga/ZSM-5 catalysts have been characterized using a broad range of techniques. According to X-ray diffraction (XRD) patterns, the MFI zeolite phase was observed in both ZSM-5 and Ga/ZSM-5 before and after the catalytic tests (Fig. S8, SI), suggesting that Ga did not modify the zeolite framework. STEM-HAADF and elemental mapping images of as-prepared Ga/ZSM-5 show the decoration of zeolite crystals by small-size Ga nanoparticles (Fig. 3aâc). Cutting of the sample by cryo-ultramicrotome demonstrates penetration of Ga to the distance of only about 20ânm in zeolite crystal (Figs. 3dâf, S9, SI), which could be explained by diffusion limitations for deeper penetration of Ga inside of the pores. It can be observed that other liquid metals, e.g., BiSn alloy, have lower penetration ability in comparison with Ga (Fig. S10, SI). Ga metal is highly mobile in the reactor, which results in modifying the zeolite surface and subsurface layer and thus, affecting its MTH performances. The lowest atomic radius of Ga (1.35âà ) in comparison with other liquid metals (In: 1.93âà , Sn: 2.17âà , Bi: 1.63âà ) and low viscosity of Ga (Ga: 1.016âmPaâs, In: 1.748âmPaâs) at 177â°C39 can provide the highest access to the micropores of ZSM-5 (5.4âà âÃâ5.6âà ).
We also used in situ TEM to trace the Ga species morphology in the Ga/ZSM-5 sample (Fig. S11, SI). During the activation with N2 and exposure to the methanol at the reaction temperature, the initial Ga droplets are deformed and redispersed to form smaller Ga particles or Ga-containing species on the surface and at the entrance of zeolite pores.
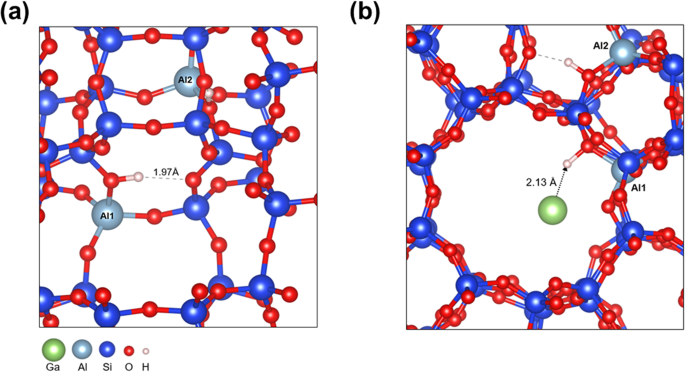
Density Functional Theory (DFT) calculations were performed to develop a description on a molecular level of the stability of small Ga clusters inside the ZSM-5 pores, and the effect of acid sites. The stability of Ga clusters with an increase of the size from 1 to 4 atoms increases, converging to the sublimation energy for bulk gallium, â290âkJ/molGa (Table S1, SI). The interaction of the small Ga clusters (Ga1âGa4) with ZSM-5 and with Silicalite-1 (pure Si form, no Al sites) was computed (Fig. 4, Figs. S12âS14, SI). Small Ga clusters interact quite strongly and specifically with the acid protons in the zeolite (Table S1, SI), with an adsorption energy of â116âkJ/molcluster for Ga1 and â143âkJ/molcluster for Ga4. The introduction of Ga also influenced the location of the acid proton. Instead of an H-bond with a neighboring O-atom, the proton now points towards the large pore to interact with Ga (Fig. 4). The adsorption energy is much weaker in the Silicalite-1, e.g., â58âkJ/molcluster for Ga1 (Table S1, SI). This specific interaction of Ga atoms and small clusters with acid sites of the zeolite can explain the partial penetration of Ga in the pores of zeolite.
The deactivation of zeolite catalyst in MTH is mainly ascribed to the carbonaceous deposition which deactivates acid sites and blocks the entrance of micropores. TG analysis (Figs. 2Ñ, S15, SI) performed on spent ZSM-5, Ga/ZSM-5 and Ga+ZSM-5 after 20âh on stream shows two stages of the weight loss (10âwt% in total) in ZSM-5_20h at about 100 and 550â°C, which can be ascribed according to the literature40 to the desorption of water and burning of condensed the graphitic coke species, respectively. Ga/ZSM-5_20h and Ga+ZSM-5_20h also show the formation of graphitic coke, however, the weight loss is about ~4 times lower than that for ZSM-5_20h. This suggests that Ga can suppress the formation of coke species over ZSM-5 and slow catalyst deactivation.
This conclusion has been further supported by N2 adsorption on ZSM-5 and Ga+ZSM-5 (Fig. 2d). The ZSM-5 after N2 activation at 450â°C for 1âh possesses a surface area of 339âm²/g, while the Ga+ZSM-5 and Ga/ZSM-5 samples show a decrease in the surface area to 266 and 160âm²/g, respectively, due to the introduction of Ga species into the pores of zeolite and dilution effect of zeolite with metallic gallium. Coke deposition over ZSM-5 after 20âh resulted in a significant decrease in the surface area to 62âm²/g. In contrast, the surface area is almost retained on Ga+ZSM-5_20h and Ga/ZSM-5_20h.
Given that the addition of Ga improves the catalytic stability and prevents the coke deposition of ZSM-5 catalysts in MTH, it is hypothesized that Ga could be also used to regenerate the coked ZSM-5 catalyst after methanol conversion. To verify this assumption, we have collected the deactivated ZSM-5 after a 20-h reaction, mixed it with Ga and tested it in the MTH reaction (Fig. S16, SI). It is observed that after the treatment with Ga, the deactivated ZSM-5 recovered its activity and showed ~100% methanol conversion for 4âh. Thus, we may assume that mobile Ga can remove coke species and liberate acid sites in MTH.
The additional visual evidence that liquid metal could suppress carbon deposition was supported by HAADF imaging and elementary analysis for both ZSM-5 and Ga/ZSM-5 used after 20âh. Figures 5 and S17, SI show a uniform distribution of carbon species in the crystal of ZSM-5_20h zeolite. It is interesting to note that in the presence of Ga, carbon is mainly localized at the intercrystalline zone mixed with Ga nanoparticles (Figs. 5b,e, S18, SI). Microscopy analysis of deactivated ZSM-5 after Ga treatment shows a significant change in the distribution of carbon in comparison with the parent ZSM-5_20h sample (Figs. 5c,f, S19, SI). There is more carbon localized in the intercrystalline zone between zeolite crystals together with Ga. It is interesting to note that large Ga particles present in the sample after treatment also contain carbon on the surface (Fig. S20, SI) indicating the high affinity of carbon to metallic Ga.
The localization of carbon in the intercrystalline zone with Ga after the reaction could be due to the extraction of coke from the zeolite, but this hypothesis is less likely due to the limited penetration capacity of Ga into the zeolite pores. Another explanation could be a stable catalytic performance mainly in this intercrystalline zone, which explains the coke generation. The role of Ga could involve continuous regeneration of acid sites in this area through the elimination of coke species until Ga and acid sites are completely saturated by carbon.
This hypothesis that Ga prevents the coke deposition by promoting the desorption of aromatics has been further checked by in situ diffuse reflectance infrared Fourier transform (DRIFT) spectra for ZSM-5 and Ga/ZSM-5 samples. Variable temperature IR spectroscopy has been carried out in the temperature range of 30â225â°C by using in situ IR cell (Fig. S21, SI). Adsorption of methanol on the catalyst surface with subsequent heating results in a gradual increase of the peak intensity at 1453âcmâ1 assigned earlier to aromatic C=C stretching vibrations (Fig. S22, SI)41,42. It should be noted that Ga/ZSM-5 catalyst does not show the formation of this peak.
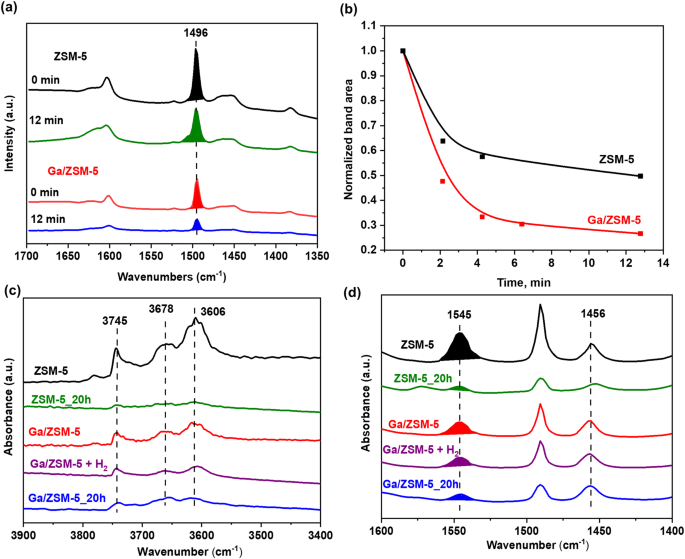
In addition, the pre-adsorption of toluene as a model aromatic molecule over ZSM-5 and Ga/ZSM-5 shows peaks at 2800â3100âcmâ1 related to C-H stretching and 1300â1500âcmâ1 related to CâC stretching in the aromatic ring and asymmetric and symmetric bending vibrations of the methyl group (Fig. 6a). The amount of toluene adsorbed is significantly lower over Ga/ZSM-5 in comparison with ZSM-5 with fast desorption of toluene in the flow of Ar in comparison with less significant desorption over ZSM-5 (Fig. 6b). These results demonstrate that the presence of Ga suppresses the adsorption of the aromatic products and enhances their desorption once they are formed in the zeolite catalyst in the MTH reaction, thus enhancing its stability.
In situ toluene desorption DRIFT spectra over ZSM-5 and Ga/ZSM-5 catalysts at room temperature for 0 and 12âmin (a). The residual percentage of toluene according to the peak-intensity changes (signal at ~1496âcmâ1) over ZSM-5 and Ga/ZSM-5 catalysts for 0â12âmin (b). FTIR spectra of activated and spent ZSM-5 and Ga/ZSM-5 catalysts with and without H2 treatment: region of hydroxyl groups vibrations (c) and region of pyridine adsorption vibrations (d).
The effects of gallium on the acid sites in ZSM-5 and Ga/ZSM-5, their concentration and strength, have been determined by pyridine-FTIR (Fig. 6c, d and Table S2, SI). The parent ZSM-5 contains intense IR adsorption bands of Brønsted acid groups (3606âcmâ1) associated with the framework Si-OH-Al bridges, isolated external silanols (3745âcmâ1) and Al-OH groups (3678âcmâ1)43. The intensity of all OH bands decreases considerably when the ZSM-5 is modified with Ga (Ga/ZSM-5). The Py adsorption on ZSM-5 results in the appearance of peaks corresponding to Brønsted (BAS, 1545âcmâ1) and Lewis acid sites (LAS, 1456âcmâ1) with concentrations of 0.82 and 0.22âmmol/g, respectively (Fig. 6d). By introducing Ga, the concentration of BAS is almost halved without a significant effect on the amount of LAS. The amount of acid sites decreases almost by a factor of 4 over ZSM-5 after 20âh of the reaction, whereas Ga/ZSM-5_20h has only 1.6 times less BAS and the same number of LAS when compared with the initial Ga/ZSM-5 catalyst.
The decrease in the amount of Brønsted acid sites after the introduction of Ga could be attributed to the oxidation of Ga to cationic Ga species (Ga+, GaO+) which should be accompanied by the generation of hydrogen. Treatment of ZSM-5 by Ga in the batch reactor at 250â°C under a 10âbar N2 atmosphere shows the formation of 1.6âmmol/g of hydrogen, which could be formed by partial oxidation of Ga with acid sites of zeolite and water in zeolite (Figs. S23 and S24, SI). Usually, the introduction of Ga by conventional impregnation results in the appearance of Lewis acid sites due to the presence of cationic Ga. The absence of new Lewis acid sites (Fig. 6d) could be explained by the interaction of formed Lewis sites with atoms of Ga in the pores resulting in their deactivation and possibly plugging of zeolite pores by Ga clusters. Indeed, according to DFT modeling penetration of metallic Ga inside of the pores could be in the form of small clusters.
Figure S25, SI shows the Ga 3d X-ray Photoelectron Spectroscopy (XPS) spectra of the Ga catalysts before and after the reaction. Compared to Ga2O3/ZSM-5 containing only Ga2O3, both gallium oxide and metallic gallium are present in Ga+ZSM-5 after physical mixing due to the oxidized surface of metallic Ga. The amount of oxidized Ga phase increases after the reaction for both Ga+ZSM-5 and Ga/ZSM-5, which could be assigned to the oxidation of Ga phase by generated water. Hydrolysis of gallium cations or oxidation of metallic Ga on the surface could result in the formation of gallium oxide. The partial oxidation of Ga can be also observed by analysis of the heat flow during TG analysis (Fig. S4, SI). The negative peak at 33â°C corresponds to the melting of Ga in the sample, which is accompanied by an exothermic peak at 470â°C due to the oxidation of Ga to oxide. The reaction in the N2 atmosphere makes these peaks less noticeable, however, they are even more obvious in the presence of H2 indicating the reduction of Ga. These results, however, support our assumption that in addition to carbon deposition, the deactivation of Ga-modified ZSM-5 could be also induced by the oxidation of Ga by water during the catalytic reaction. It should suppress the regeneration effect of metallic Ga on the acid sites. This also suggests that the MTH stability potentially could be further enhanced by co-feeding methanol with hydrogen. H2-TPR of Ga2O3/ZSM-5 shows reduction starting only at about 400â°C (Fig. S26, SI). However, Ga/ZSM-5 shows reduction already at ~360â°C corresponding to smaller gallium oxide particles or gallium oxide over the surface of metallic Ga with a broad peak till 650â°C. Note that the catalytic stability in the presence of hydrogen has been significantly improved for both Ga/ZSM-5 and Ga+ZSM-5 (Fig. 2). XPS analysis confirms a significant increase in the contribution of metallic Ga after the reaction in the presence of hydrogen in comparison with the pure oxide phase over Ga2O3/ZSM-5, which supports our hypothesis about the importance of metallic Ga for stable catalytic performance (Fig. S25, SI). It is interesting to note that cationic Ga is most probably not affected by hydrogen due to the same amount of adsorbed Py after pretreatment of the Ga/ZSM-5 catalyst in H2 at 450â°C (Fig. 6c,d).
Based on these results, we could assume that oxidized Ga should not contribute significantly to the increase in the stability of ZSM-5 during MTH reaction. However, these species affect the selectivity of the reaction in N2 atmosphere, resulting in an increased yield of aromatics for Ga2O3/ZSM-5 and Ga/ZSM-5 compared to ZSM-5, which could be the result of the dehydrogenation activity on Ga oxide (Fig. 2). Ga+ZSM-5 provides lower selectivity to aromatics most probably due to less uniform distribution of Ga in the sample and lower content of oxidized Ga in this case. Interestingly, the hydrogenation activity on Ga/ZSM-5 and Ga+ZSM-5 is considerably enhanced in the presence of hydrogen with higher paraffins production compared to ZSM-5 and Ga2O3/ZSM-5 (Fig. 2). This could indicate hydrogenation behavior of cationic Ga in ZSM-5 or oxidized Ga on the Ga metal surface.
According to TEM and FTIR results, metallic Ga localized over the external surface of zeolite and at the entrance of the pores effectively promotes more stable methanol conversion in MTH process by slowing deposition and facilitating the desorption of carbon species. The internal acid sites should contribute less to the catalytic performance due to blockage of the pores by liquid Ga and their deactivation by reaction with Ga. At the same time, Ga at the external surface of zeolite provides continuous refreshing of the acid sites in the intercrystallite voids by removing coke species and liberating the acid sites. This effect could be attributed to the high affinity of metallic Ga to carbon species (Fig. 5), which has been observed in the literature for different applications44,45. It should result in the pushing out carbon species and the release of acid sites of ZSM-5. The interaction of Ga with acid sites of ZSM-5 according to DFT modeling should also promote the desorption of carbon species (Fig. 4). It is also supported by FTIR spectroscopy indicating a decrease in the strength of interaction between acid and adsorbed organic compounds in the presence of Ga. The dynamic nature of Ga promotes the migration of carbon at the interface of zeolite crystals, which can be observed by microscopy. This method of stability enhancement in the presence of liquid metal may act as an alternative route to design more stable high-temperature processes for zeolite catalysts.
We have demonstrated boosted stability of the MTH process on a ZSM-5 catalyst conducted in the presence of liquid metal. Multiple characterizations revealed that liquid Ga is localized in the intercrystalline area and partially penetrates in the ZSM-5 micropores, modifies the zeolite acidic properties, slows down carbon deposition and facilitates the desorption of aromatic molecules. Moreover, it has been found that co-feeding with hydrogen could enhance the MTH lifetime by a factor of ~14 compared to ZSM-5. We believe that the facile mixing of the zeolite catalyst with low-melting-point metal can act as a powerful promoter for the MTH process, which could also open an alternative route to the design and preparation of a more efficient zeolite catalyst system for other high-temperature reactions.