We explore the challenges and opportunities for electrochemical energy storage technologies that harvest active materials from their surroundings. Progress hinges on advances in chemical engineering science related to membrane design; control of mass transport, reaction kinetics and precipitation at electrified interfaces; and regulation of electrocrystallization of metals through substrate design.
Cost-effective energy storage systems and autonomous robotics have emerged nearly simultaneously in the past three decades as important technological challenges for researchers worldwide1,2. A solution to the former that is capable of scaling to meet the extraordinarily large global need is conventionally viewed as a requirement for the âelectrify everythingâ pathway to reducing humanityâs carbon footprint. The search for solutions to the latter is at a relatively early stage and is driven by emergent opportunities for autonomous systems with an efficient, embedded energy supply (here termed a self-sufficient energy system) that enables computers to take direct actions in the physical world. Apart from advances made in autonomous electric vehicles and drones, this search has largely overlooked the need for the self-sufficient energy storage solutions required for truly autonomous systems. In this Comment, we highlight opportunities in chemical engineering, materials science and related fields provided by energy storage approaches capable of continuously harvesting reagents from their surroundings as potential solutions in both areas. Among the various possibilities, rechargeable self-sufficient metalâair battery (SMAB) systems that use Earth-abundant metals (for example, Al, Fe, Na and Zn) at the anode are likely to attract greatest interest3. SMABs based on any of these anodes can meet the cost, scale, simplicity and performance characteristics required in applications â all are challenged by the inability to reversibly charge and discharge the cells in ambient air. The challenge is compounded by the fact that fundamental issues associated with recharge of the most actively studied metal anodes (such as Li, Na and Zn) are exacerbated by new chemical kinetics, solution thermodynamics and mass transport challenges at the battery anode, electrolytes and cathode.
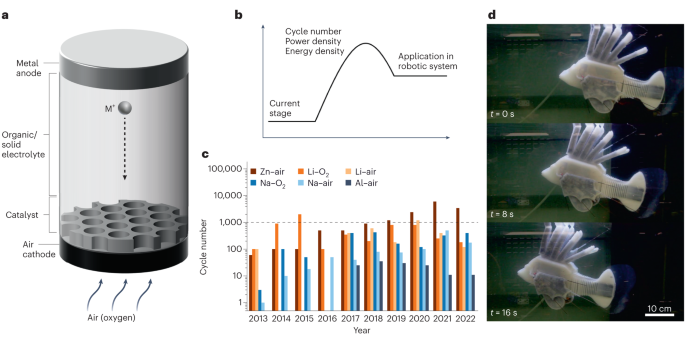
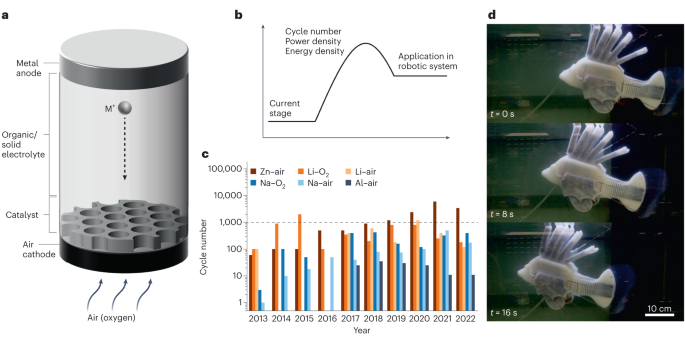
In a metalâair battery (Fig. 1), the active cathode material (typically molecular oxygen or carbon dioxide) first dissolves from an ambient gaseous fluid into a nominally stagnant electrolyte, and thereafter diffuses to the surface of a porous, electronically conductive substrate where it undergoes an electrochemical reaction with metal ions to form mixtures of metal oxide, peroxide, superoxide, hydroxides, carbonate and bicarbonate at the substrate4,5,6,7. Poor solubility of any of these reaction products retards the anodic reaction by blocking mass, ion and/or electron transport at the cathode. Nucleophilic reaction products at the cathode may also undergo chemical reactions with electrolyte components to deplete the electrolyte. Finally, dissolved gases may diffuse across the interelectrode space to undergo irreversible, parasitic reactions with the anode, depleting the anode material and precipitating passivating interphases that limit cathodic reactions. A consequence is that it has proven difficult to create metalâair battery cells that achieve practical energy densities close to those one would predict from the theoretical electrode reactions, and more difficult still to create cells that operate reversibly over the hundreds to thousands of chargeâdischarge cycles to achieve sufficient amortization of the acquisition costs for metalâair batteries to be competitive in the applications noted (Fig. 1).
a, Schematic illustration of the SMAB system in an atmospheric environment. b, Practical barriers including cycle number, power density and energy density to the application of SMABs in autonomous robotic systems. c, Development of cycle number of different metalâair and metalâoxygen batteries over the period 2013â2022. The horizontal dashed line indicates 1,000 cycles of the cycle number. d, An autonomous robotic system. M+, metal ion. Panel d adapted with permission from ref. 2, Springer Nature Ltd.
A compromise adopted in the literature is to replace air as the active ingredient in a SMAB cathode with pure oxygen. Although reasonable for understanding materials science and electrochemical processes at the scale of the battery electrode, the added weight, cost and complexity (for example, safety) of the equipment needed to separate oxygen from ambient air or to integrate a dedicated oxygen supply (for example, an oxygen tank for mobile storage or supply line for stationary storage) eliminates most, if not all, practical benefits of the metalâair battery. Nonetheless, even under this simplified scenario, recharge of any metalâoxygen battery requires the electrodeposition of metals such as Li, Na, Al and Zn â a process that is known to be fundamentally challenged by chemical, morphological, hydrodynamic and mechanical instabilities that become progressively more difficult to overcome as the charge current density and storage capacity rises8,9. Thus, to realize practically relevant SMABs â batteries capable of sustaining operation at areal capacities of 10 mAh cmâ2 or higher10, of maintaining high material utilization ratios at depth of discharge of 50% or greater, and of delivering at least 1,000 cycles of trouble-free chargeâdischarge operations â breakthroughs are required in multiple areas relevant to chemical engineering science.
Concepts from mass transport, thermodynamics and chemical kinetics are critical for the design of the cathode in any SMAB. From the design and scale-up of air-permeable membranes with high oxygen or carbon dioxide selectivity â that is, the development of catalysts that promote the oxygen evolution reaction (OER) and resist poisoning by impurities in the air stream or insoluble redox reaction products at the cathode, and the formulation of electrolytes with high room-temperature ion conductivities and the right balance of solvating power for the active ions and cathode reaction products â chemical engineers and the application of chemical engineering concepts will play a critical role in the development of practical SMABs. The ideal air-permeable membranes, for example, must not only be able to facilitate air transport at high flow with minimum compressor costs, but must remain chemically and mechanically stable in direct, long-term contact with humid, ambient air. They must, ideally, also permit selective oxygen passage while preventing the infiltration of undesirable molecules such as nitrogen or water into the battery electrolyte. Promising membrane materials such as polysulfones, fluorinated polysiloxanes, polyethers and polymers containing Trögerâs base have been reported as candidates for this purpose11. Suitable OER catalysts must likewise not only be cost effective, but tolerant of oxygen and resistant to poisoning by nucleophilic peroxide species formed at the battery cathode and by impurities present in ambient air. Depending on the cathode design, the membrane and electrocatalyst are in contact with the electrolyte, which introduces additional challenges. For example, while liquid (salt-in-solvent) electrolytes based on high Gutmann donor number solvents4 with wide redox stability windows offer multiple benefits, parasitic oxidation reactions at the cathode and solvent loss due to the combined effects of heat generated in the cell and evaporation via the open, porous architecture of the cathode pose major challenges. This has motivated research into electrolytes with gradient transport properties (for example, solid-like near the anode and liquid-like at the cathode)1, because of their potential to support high oxygen permeabilities at the cathode while limiting crossover of the molecular species dissolved in the electrolyte to the anode as well as the out-of-plane growth of metal electrodeposits at the anode during battery recharge.
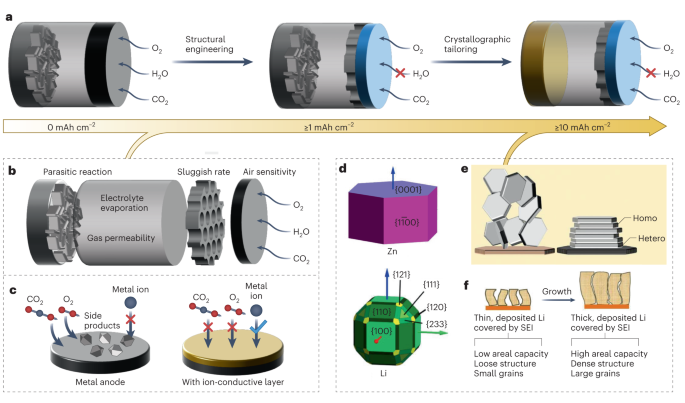
Chemical engineering concepts are likewise important in the design of the anode of a SMAB. It is understood, for example, that to achieve repeated trouble-free charging of any metal anode, the growth of metal deposits must be suppressed along the surface normal of the conductive substrate (that is, current collector) where the deposition occurs. In practice, this requires a mix of electrochemical engineering of the battery cell and materials design for the electrodes, electrolytes and interphases (Fig. 2). Specifically, the recent decade has witnessed a rapid intensification of experiments and theoretical efforts using progressively more sophisticated methods, which show how, why and by what mechanisms the growth of metal electrodeposits occurs preferentially along the surface normal to a current collector8,12,13. Although multiple open questions remain, particularly concerning the precise interplay between the crystallographic features of metals deposited under different cell operating conditions and the electrochemical kinetics of parasitic reactions at the metalâelectrolyte interface, the consensus view is that the conceptual simplicity of the charging process â metal ions coordinated with a solvent or solid electrolyte are driven to the electrode substrate under the action of diffusion and electromigration where they are firstly de-solvated and then reduced to form the metal â belies its fundamental complexity from at least three different perspectives. First, the metal electrodes are crystalline materials with symmetry features that may be fundamentally at odds with those required to produce uniform, compact deposits at the typically planar anode geometry inside a battery cell12. It is known, for instance, that deposition of a metal electrode in which the crystal planes with the highest atom density (for example, (110) for body-centred cubic (bcc) metals such as Li (ref. 13) or (002) for hexagonal close-packed (hcp) metals such as Zn (ref. 14)) are aligned parallel to the electric field would lead to more planar and compact plating during battery recharge (Fig. 2); however, with few exceptions reported in the literature, it has been difficult to achieve this level of crystal-scale control in a battery cell14. Second, at the charging potentials, electroreduction of the metal ions rarely occurs in isolation: it typically occurs in tandem with other electrolyte components, yielding new, typically uncontrollable and unwanted material phases (solid-electrolyte interphase layers) at the same time as the metal deposits. Whether the new phases are organic or inorganic solids, liquids or gases, their interfacial transport properties are invariably worse than the metal of interest, which compromises the coating quality8. Third, a consequence of the first and second complications is that the deposition is non-uniform and anisotropic. This produces patchy, non-planar metal deposits (typically in wire-like or mossy morphologies) partly because of the MullinsâSekerka instability in which ions are preferentially transported by diffusion and electromigration toward high points on the metal surface due to enhanced electric field and concentration gradients at the peaks9,15. This diversity of failure modes has led to a very wide diversity of remedies, ranging from electrolyte to interphase to electrode designs, which improve the reversibility of metal anodes to some extent. It is rare, however, to find a single approach that meets the stringent cost, scalability and performance requirements needed to achieve practical SMAB systems based on high-areal-capacity metal anodes.
a, Schematic illustration of the development direction from irreversible metalâair batteries to stable high-capacity metalâair batteries. b, Challenges that exist in various parts of metalâair batteries under atmospheric environments. c, Artificial solid-electrolyte interphase (SEI) design for reversible air-sensitive anode materials at low areal capacity (â¤1 mAh cmâ2), such as Li and Na. dâf, Crystallographic orientations (d) and deposition of a Zn metal anode (e) and a Li metal anode (f). Figure adapted with permission from: c, ref. 3, RSC; d, ref. 13, American Chemical Society; e, ref. 14, AAAS; f, ref. 12, under a Creative Common license CC BY 4.0.
The possibility of parasitic chemical and electrochemical reactions between freshly deposited metal atoms and dissolved species in ambient air poses additional challenges for the anode design of a SMAB. There is, however, a growing body of work showing that artificial solid-electrolyte interphases created either in situ (for example, using sacrificial electrolyte additives, by ion exchange with salt additives, or by selective polymerization and crosslinking of electrolyte molecules near the metal electrode), or outside the battery cell (for example, using atomic layer deposition, LangmuirâBlodgett or other solution coating methods in chemically inert solvents8, or interface-initiated polymerization of gaseous monomers) provide an increasingly powerful arsenal of interphase design concepts able to protect a metal anode from unwanted chemical species while facilitating transport of the active ion species required for overcoming the most stubborn of the interfacial instabilities (Fig. 2). Finally, we note that the transition from laboratory-scale (anode areal capacity of 1â2 mAh cmâ2) SMABs that exhibit good reversibility to practically relevant (â¥10 mAh cmâ2; â¥1,000 trouble-free chargeâdischarge cycles) metalâair batteries demanded by autonomous robotics and stationary storage applications will require deliberate, focused attention to unique challenges associated with scale-up and control processes for battery packs. To that end, and as an important first step for accelerating progress toward scalable battery systems, we recommend discouraging the current practice in the literature of reporting electrochemical performance of isolated battery cells based on small-scale prototypes (for example, CR2032 coin cells with perforated caps). And, instead, encouraging demonstration of prototype packs composed of cells with the design features of interest in an application of interest (for example, the ability to power an autonomous robot to accomplish a desired function for a particular time)2.
In summary, stand-alone secondary metalâair batteries able to harvest active materials from their surroundings offer important solutions for stationary storage and for powering autonomous robots. Long-term reversibility of air-sensitive metal anodes; membrane designs that selectively remove water vapor and other gaseous contaminants from ambient air and at low to moderate pressure drops; OER and oxygen reduction reaction (ORR) catalysts able to operate stably at the high capacities and long cycle lives; and largely unknown design rules that govern scale-up of small-scale prototypes reported in laboratory studies to battery systems demanded in applications pose key fundamental and practical barriers to progress. We believe that the search for solutions to these challenges offers an exciting opportunity for chemical engineering innovation.